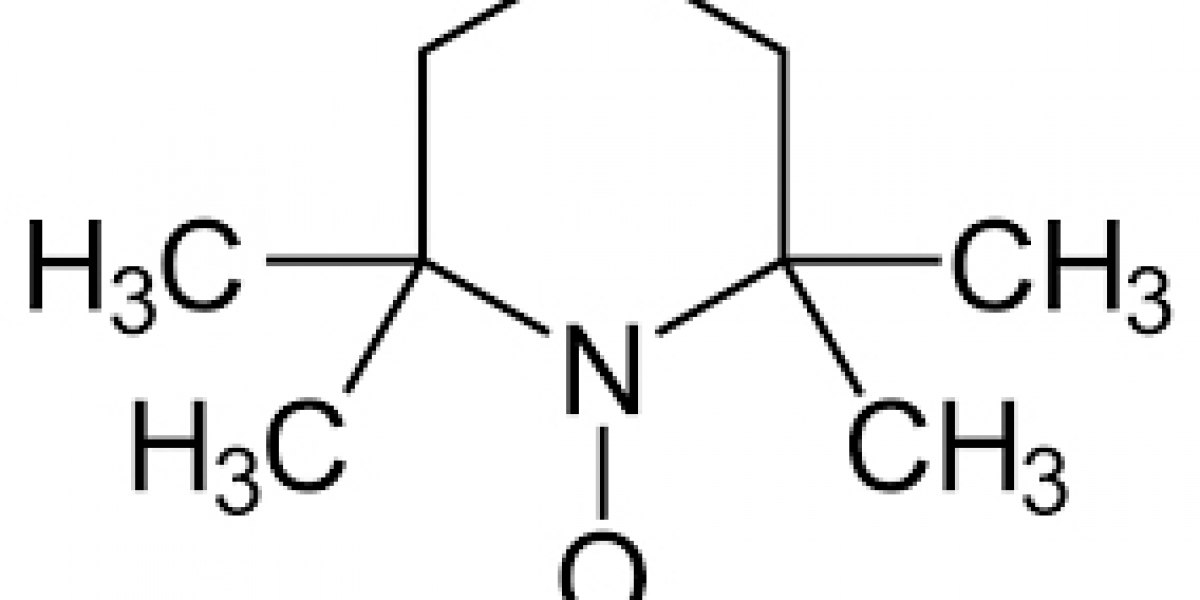
The field of organic chemistry often involves the use of specialized compounds that enable complex reactions with greater efficiency and control. One such important compound is the TEMPO Free Radical, also known by its chemical name 2,2,6,6-Tetramethylpiperidine-1-oxyl. Although it may sound technical, TEMPO is a fascinating and widely used compound in both academic and industrial chemistry.
In this article, we will explore what the TEMPO Free Radical is, its unique chemical properties, key applications, methods of preparation, and important safety considerations. Whether you're a student, researcher, or chemistry enthusiast, this blog aims to provide a clear understanding of TEMPO without unnecessary jargon.
What is TEMPO Free Radical?
TEMPO Free Radical (2,2,6,6-Tetramethylpiperidine-1-oxyl) is a stable nitroxyl radical. Unlike most radicals, which are highly reactive and short-lived, TEMPO is exceptionally stable due to its molecular structure. This stability makes it a useful tool in a variety of oxidation and catalytic processes.
Chemical Formula: C9H18NO
Molecular Weight: 156.25 g/mol
Appearance: Red-orange crystalline solid
CAS Number: 2564-83-2
The compound’s stability and reactivity arise from the presence of the nitroxyl group (–N–O•) in its ring structure, which enables it to participate in controlled oxidation reactions.
Chemical Structure and Stability
TEMPO consists of a six-membered piperidine ring substituted with four methyl groups at the 2 and 6 positions, along with a nitroxyl radical at the nitrogen atom. The presence of bulky methyl groups helps stabilize the radical center, making TEMPO one of the rare examples of a stable free radical.
This structural stability is a key reason why 2,2,6,6-Tetramethylpiperidine-1-oxyl is so valuable in organic synthesis and materials science.
Key Applications of TEMPO Free Radical
TEMPO is not just a chemical curiosity—it has practical uses in various fields. Below are some of its main applications:
1. Oxidation Reactions
One of the most well-known uses of TEMPO is in the selective oxidation of alcohols to aldehydes or ketones. It serves as a mild, efficient catalyst that enables chemists to avoid harsh oxidizing agents.
2. Polymer Chemistry
TEMPO is used in the synthesis and modification of polymers. It plays a role in controlled radical polymerization techniques, such as nitroxide-mediated polymerization (NMP), which allows for the creation of polymers with specific architectures and properties.
3. Cellulose Modification
In materials science, TEMPO is employed to oxidize cellulose fibers, leading to the production of nanocellulose. This process is eco-friendly and widely used in the development of biodegradable materials and lightweight composites.
4. Redox Mediator
TEMPO is used as a redox mediator in electrochemical applications, including energy storage devices like batteries and supercapacitors. It helps facilitate electron transfer without undergoing permanent chemical change.
5. Research Tool
In spectroscopy and magnetic resonance studies, TEMPO acts as a spin label. Its unpaired electron makes it detectable in Electron Paramagnetic Resonance (EPR) spectroscopy, helping scientists study molecular motion and interactions.
How is TEMPO Synthesized?
The synthesis of TEMPO Free Radical (2,2,6,6-Tetramethylpiperidine-1-oxyl) generally involves a multi-step process starting from 2,2,6,6-tetramethylpiperidine. The key step is the oxidation of the amine to a nitroxyl radical.
A common laboratory method includes:
Initial formation of the piperidine ring with four methyl groups.
Oxidation using reagents like sodium hypochlorite or hydrogen peroxide in the presence of a catalyst.
This process produces the stable red-orange solid that can be purified and used directly in various applications.
Safety and Handling Information
While TEMPO is relatively stable compared to other free radicals, proper care should still be taken when handling it.
Hazards
Irritant: Can cause skin and eye irritation on contact.
Combustible: May support combustion under certain conditions.
Toxicity: Avoid ingestion and inhalation. TEMPO can be harmful if swallowed or inhaled in large amounts.
Storage Recommendations
Store in a tightly sealed container in a cool, dry place.
Keep away from heat and direct sunlight.
Avoid contact with strong acids and reducing agents.
Protective Measures
Use gloves and safety goggles in the laboratory.
Work in a well-ventilated area or fume hood.
Follow standard laboratory safety protocols.
Always consult the Material Safety Data Sheet (MSDS) before working with TEMPO.
Environmental Considerations
Although TEMPO is used in green chemistry approaches, such as selective oxidation, it is important to manage its disposal properly. Do not release it into water systems or soil. Collect and dispose of TEMPO waste according to local hazardous waste disposal regulations.
Conclusion
TEMPO Free Radical (2,2,6,6-Tetramethylpiperidine-1-oxyl) is a remarkable compound that stands out for its unique stability and wide-ranging applications in modern chemistry. From its role in clean oxidation reactions to its importance in polymer and materials science, TEMPO continues to be an essential reagent in both academic research and industrial processes.
Understanding its chemical structure, safe handling, and practical uses allows researchers and professionals to fully harness its potential. As green chemistry continues to grow, the demand for versatile and efficient reagents like TEMPO is likely to increase. Methyl vinyl ketone manufacturers play a crucial role in supplying high-purity chemicals for industrial and research applications.